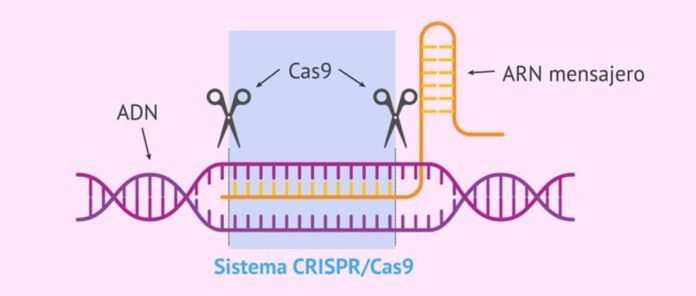
“CRISPR-Cas systems” in bacteria and viruses identify and destroy invading viral sequences. It is bacterial and archaeal immune system for protection against viral infections. In 2012, CRISPR-Cas system was recognised as a ゲノム editing tool. Since then, wide range of CRISPR-Cas systems have been developed and have found applications in areas such as in gene therapy, diagnostics, research and crop improvement. However, currently available CRISPR-Cas systems have limited clinical use due to frequent occurrences of off-target editing, unexpected DNA mutations and inheritable problems. Researchers have recently reported a novel CRISPR-Cas system that can target and destroy mRNA and タンパク質 associated with different genetic diseases more accurately without off-target impact and inheritable problems. Named Craspase, it is the first CRISPR-Cas system that shows タンパク質 editing function. It is also the first system that can edit both RNA and タンパク質. Because Craspase overcomes many limitations of existing CRISPR-Cas systems, it has potential to revolutionise gene therapy, diagnostics and monitoring, biomedical research, and crop improvement.
“CRISPR-Cas system” is natural immune system of bacteria and archaea against viral infections that identifies, binds and degrades the sequences in the viral gene to protect. It consists of two parts – bacterial RNA transcribed from the viral gene incorporated in the bacterial genome after first infection (called CRISPR, this identifies the target sequences of the invading viral genes) and an associated destroyer タンパク質 called “CRISPR associated タンパク質 (Cas)” which binds and degrades the identified sequences in the viral gene to protect the bacteria against viruses.
クリスパー stands for “clustered regularly interspaced short palindromic repeats”. It is transcribed bacterial RNA characterised by palindromic repeats.
回文反復 (CRISPR) は、以下の配列で最初に発見されました。 E. 大腸菌の in 1987. In 1995, Francisco Mojica observed similar structures in archaea, and it was he who first thought of these as a part of the immune system of bacteria and archaea. In 2008, it was experimentally demonstrated for the first time that the target of the immune system of bacteria and archaea was foreign DNA and not mRNA. The mechanism of identification and degradation viral sequences suggested that such systems could be used as a tool for ゲノム編集. Since its recognition as a genome editing tool in 2012, CRISPR–Cas system has come a very long way as a firmly established standard 遺伝子編集 system and has found a wide range of applications in biomedicine, agriculture, pharmaceutical industries including in clinical gene therapy1,2.
広範囲の CRISPR-Cas systems are already identified and currently available for monitoring and editing DNA/RNA sequences for research, drug screening, diagnostics and treatments. The current CRISPR/Cas systems are divided into 2 classes (Class 1 and 2) and six types (Type I to XI). Class 1 systems have multiple Cas タンパク質 which need to form a functional complex to bind and act on their targets. On the other hand, Class 2 systems have only one large Cas タンパク質 for binding and degrading target sequences which makes Class 2 systems easier to use. Commonly used Class 2 systems are Cas 9 Type II, Cas13 Type VI, and Cas12 Type V. These systems may have undesired collateral effects I.e., off-target impact and cytotoxicity3,5.
遺伝子治療 based on current CRISPR- Cas systems have limited clinical use because of frequent occurrences of off-target editing, unexpected DNA mutations, including big DNA fragment deletions and large DNA structural variants at both on-target and off-target sites that leads to cell deaths and other inheritable problems.
クラスパーゼ (または CRISPR 誘導カスパーゼ)
Researchers have recently reported a novel CRISPER-Cas system which is a Class 2 Type III-E Cas7-11 system associated with a caspase-like タンパク質 hence named クラスパーゼまたは CRISPR 誘導型カスパーゼ 5 (Caspases are cysteine proteases that play key role in apoptosis in breaking down cellular structures). It has potential applications in areas like gene therapy and diagnostics. Craspase is RNA-guided and RNA-targeted and do not get involved with the DNA sequences. It can target and destroy mRNA and タンパク質 associated with different genetic diseases more accurately without off-target impact. Thus, elimination of genes associated with diseases is possible by cleavage at mRNA or protein level. Also, when linked with specific enzyme, Craspase can also be used to modify functions of proteins. When its RNase and protease functions are removed, Craspase becomes deactivated (dCraspase). It has no cutting function but binds with RNA and protein sequences. Therefore, dCraspase can be used in diagnostics and imaging to monitor and diagnose diseases or viruses.
Craspase is the first CRISPR-Cas system that shows protein editing function. It is also the first system that can edit both RNA and protein. Its 遺伝子編集 function comes at minimal off-target effects and no inheritable problems. Hence, Craspase is likely to be safer in clinical use and therapeutics than other currently available CRISPR- Cas systems 4,5.
Craspase は既存の CRISPR-Cas システムの多くの制限を克服するため、遺伝子治療、診断とモニタリング、生物医学研究、および作物の改良に革命をもたらす可能性があります。 臨床試験で安全性と有効性を証明する前に、細胞内の疾患原因遺伝子を正確に標的とする信頼性の高い送達システムを開発するには、さらなる研究が必要です。
***
参照:
- Gostimskaya、I. CRISPR–Cas9: その発見の歴史とゲノム編集におけるその使用の倫理的考慮事項。 生化学モスクワ 87、777–788 (2022)。 https://doi.org/10.1134/S0006297922080090
- チャオ・リー ら 2022. CRISPR/Cas ゲノム編集のための計算ツールとリソース。 ゲノミクス、プロテオミクス、バイオインフォマティクス。 24 年 2022 月 XNUMX 日にオンラインで入手可能になります。DOI: https://doi.org/10.1016/j.gpb.2022.02.006
- ヴァン・ベルジュー、SPB、サンダース、J.、ロドリゲス・モリーナ、A. 他RNA を標的とする CRISPR-Cas システム。 Nat Rev Microbiol 21、21–34 (2023)。 https://doi.org/10.1038/s41579-022-00793-y
- 胡春儀 ら 2022. クラスパーゼは、CRISPR RNA 誘導性の RNA 活性化プロテアーゼです。 化学。 25 年 2022 月 377 日。第 6612 巻、第 1278 号。1285-XNUMX ページ。 土井: https://doi.org/10.1126/science.add5064
- Huo, G.、Shepherd, J. & Pan, X. Craspase: 新しい CRISPR/Cas デュアル遺伝子エディター。 機能的および統合的ゲノミクス 23、98 (2023)。 公開日: 23 年 2023 月 XNUMX 日。DOI: https://doi.org/10.1007/s10142-023-01024-0
***